PENTAX Medical highlights new products supporting total approach to disease management at UEGW 2017
PENTAX Medical introduces key new products in endoscopic hygiene and therapeutics.
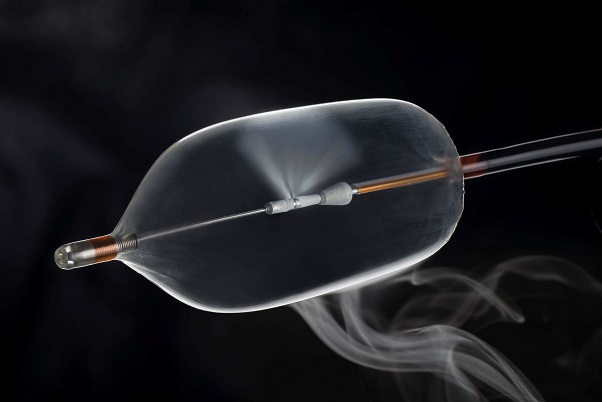
The newC2 CryoBalloon™ Ablation System offers a simple, cost effective and fast solution for the treatment of pre-cancerous lesions of the esophagus.
Hamburg, 20th December 2017 – PENTAX Medical, a leading company in the endoscopic field, highlights how it has developed a range of screening, diagnostic and therapeutic endoscopic solutions for the complete management of the most common conditions encountered by healthcare providers. This follows extensive market research by its R&D department which surveyed clinicians globally.
Colorectal cancer, Barrett’s esophagus, squamous cell carcinoma, IBD and biliopancreatic diseases can all be effectively diagnosed and treated via endoscopic procedures. To provide a comprehensive endoscopic clinical pathway supported by a single supplier, PENTAX Medical has added new products to its range of integrated technologies. A number of these new endoscopic products were recently demonstrated and presented at UEGW 2017. These include:
The new DECTM Duodenoscope, enhancing PENTAX Medical’s biliopancreatic solution.
Recently launched, this unique new duodenoscope with a Disposable Elevator Cap was demonstrated live from Evangelisches Krankenhaus, Düsseldorf, Germany, by Prof. Horst Neuhaus and his team. Representing a breakthrough for infection control, the new DECTM Duodenoscope increases reprocessing operational efficiency, while ensuring reliable diagnostic and therapeutic performance for endoscopic retrograde cholangio-pancreatography (ERCP) procedures.
New therapeutic devices completing PENTAX Medical’s GI solutions.
Seen for the first time on the PENTAX Medical booth, the C2 CryoBalloon™ Ablation System offers a simple, cost effective and fast solution for the treatment of pre-cancerous lesions of the esophagus. Esophageal cancer is the sixth leading cause of cancer deaths in the world and the CryoBalloon™ Ablation System delivers an effective therapeutic solution, enabling early intervention for this common disease. It can immediately ablate unwanted tissue in the gastrointestinal tract by delivering a targeted dose of cryogen (extreme cold). This means that interventional endoscopists can treat Barrett’s disease and squamous dysplasia with less impact on the patient than using hot ablation, resulting in better patient outcomes and lowering the level of complications.
Another addition to the PENTAX Medical therapeutic portfolio supporting better patient care is the HemoStat WideCup™. This new bipolar hot hemostasis forceps device is contributing to early treatment of gastric cancer. Highly innovative, it is designed to greatly simplify the control of bleeding to ensure precise, efficient and safer endoscopic therapy. The addition of this new hemostatic solution, means that PENTAX Medical now has the most comprehensive colonoscopy product offering, supporting the improvement of the key criteria determined by the standards of Quality in Colonoscopy.
Supporting Colorectal cancer screening, “PENTAX Medical scientific event”
The clinical value of these three key new products was also discussed in greater depth by a panel of expert speakers at the PENTAX Medical sponsored UEGW ‘Expert Dinner’, chaired by Prof. Dr. Ralf Kiesslich (Wiesbaden, Germany) and Prof. Peter Siersema (Nijmegen, Netherlands)
Videos of each of the Expert lectures can now be viewed at https://vimeopro.com/user46611207/uegw-expert-dinner-2017. These include fascinating insights into image guided endoluminal therapy in Barrett’s esophagus and advanced imaging for detection and decision making, including use of the C2 CryoBalloon, were presented by Prof. Raf Bisschops (Leuven, Belgium). Advanced endoscopy imaging and therapy in the colon, supported by PENTAX Medical’s new hemostatic solutions, was presented by Prof. Marietta Iacucci (Birmingham, UK). Focusing on the application of the new DEC™ Duodenoscope, Dr. Marc Giovannini (Marseille, France) discussed state-of-the-art biliopancreatics’ disease management and infection control.
Commenting on PENTAX Medical’s end-to-end disease management approach highlighted at UEGW 2017, Rainer Burkard, President EMEA, PENTAX Medical said, “To support the entire clinical pathway for common diseases, we have been investing significantly in R&D to provide our customers with a complete solution for image guided therapy. By addressing the most frequent conditions with our integrated tools we aim to improve the efficiency and efficacy of many endoscopic procedures. There is a real benefit to both the provider and patient, if clinicians are using the latest, most accurate systems for endoscopy, secure in the knowledge that all are engineered to the highest standards. Using the most advanced endoscopy technology throughout the clinical pathway offers patients optimum outcomes.”
About PENTAX Medical
PENTAX Medical is a division of HOYA Group. Its mission is to improve the standard of patient care and quality of healthcare delivery by providing the best endoscopic products and services with a focus on QUALITY, CLINICALLY RELEVANT INNOVATION, and SIMPLICITY. Through leading edge R&D and manufacturing, PENTAX Medical provides endoscopic imaging devices and solutions to the global medical community. Headquartered in Japan, PENTAX Medical has a worldwide focus and presence with R&D, regional sales, service, and in-country facilities around the globe.
For more information, please visit: www.pentaxmedical.com.

