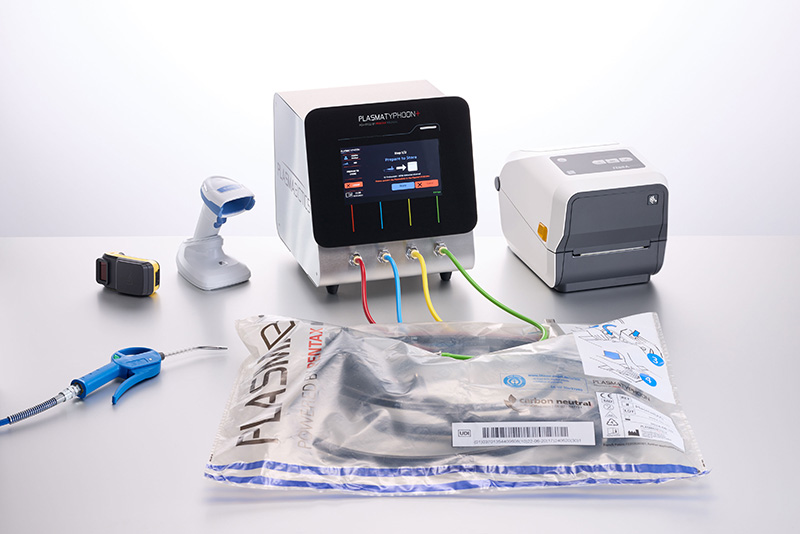
PlasmaTYPHOON™+ and PlasmaBAG™ System
REDUCE ENDOSCOPE
DRYING AND STORAGE
TO JUST 1-3 MINUTES

Boosting endoscope reprocessing to enhance patient safety and hospital efficiency
Hygiene is essential for patient safety
PENTAX Medical strives to continuously improve patient safety and infection prevention in endoscopy. That’s why we revolutionised endoscope reprocessing procedures to achieve three major goals:

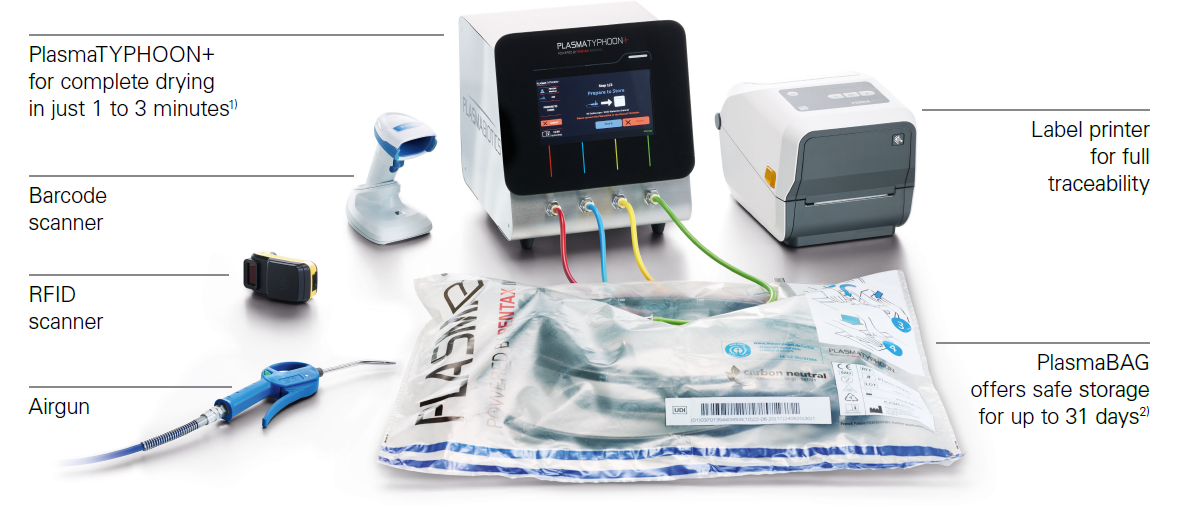
PlasmaTYPHOON™+ and PlasmaBAG™ System Ask your reprocessing staff: PlasmaTYPHOON™+ and PlasmaBAG™ system dries and stores endoscopes faster than any other system, ever.
|  |
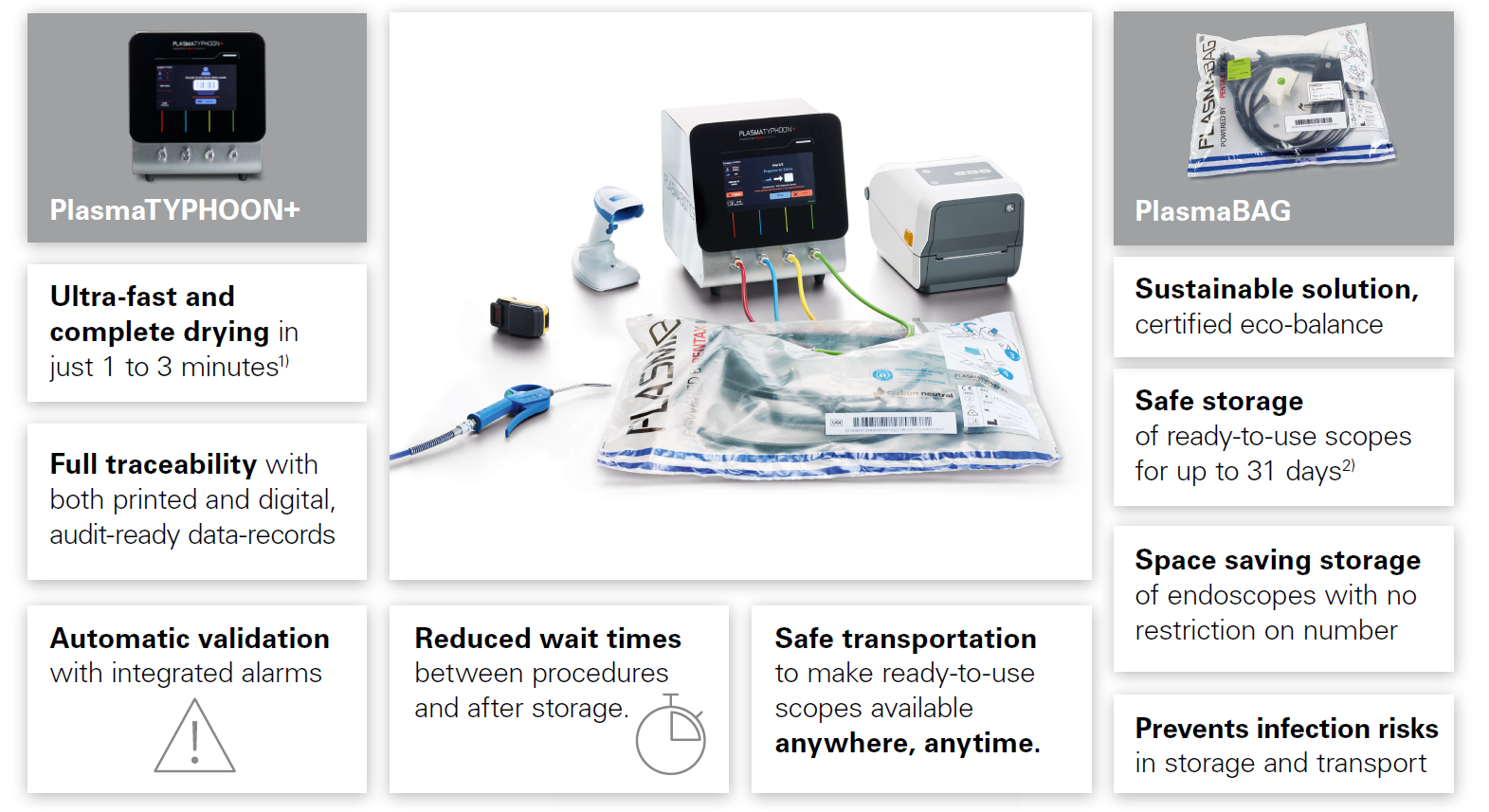
PlasmaTYPHOON™+ and PlasmaBAG™ System
Small dimensions – unlimited capacity
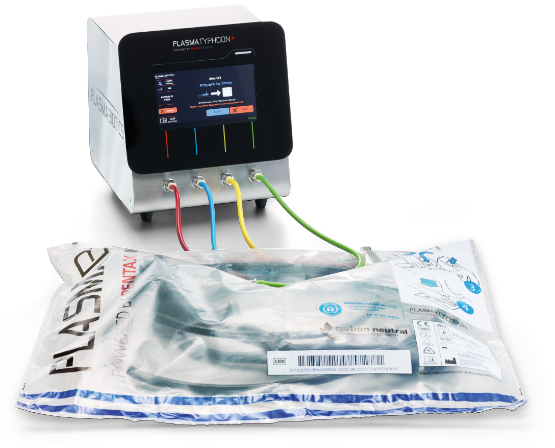
PlasmaTYPHOON™+
Reduces drying times from hours to minutes
A specific airflow process ensures each individual endoscope channel is perfectly dried:
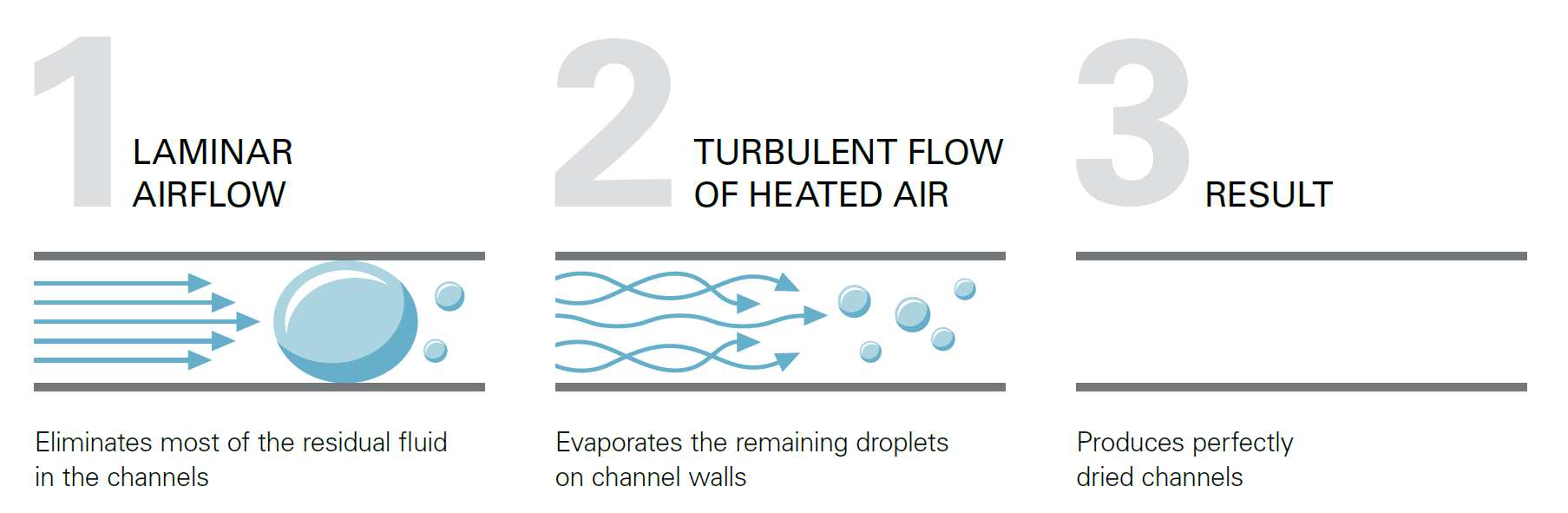
The result: Perfectly dried channels for endoscopes of all major brands
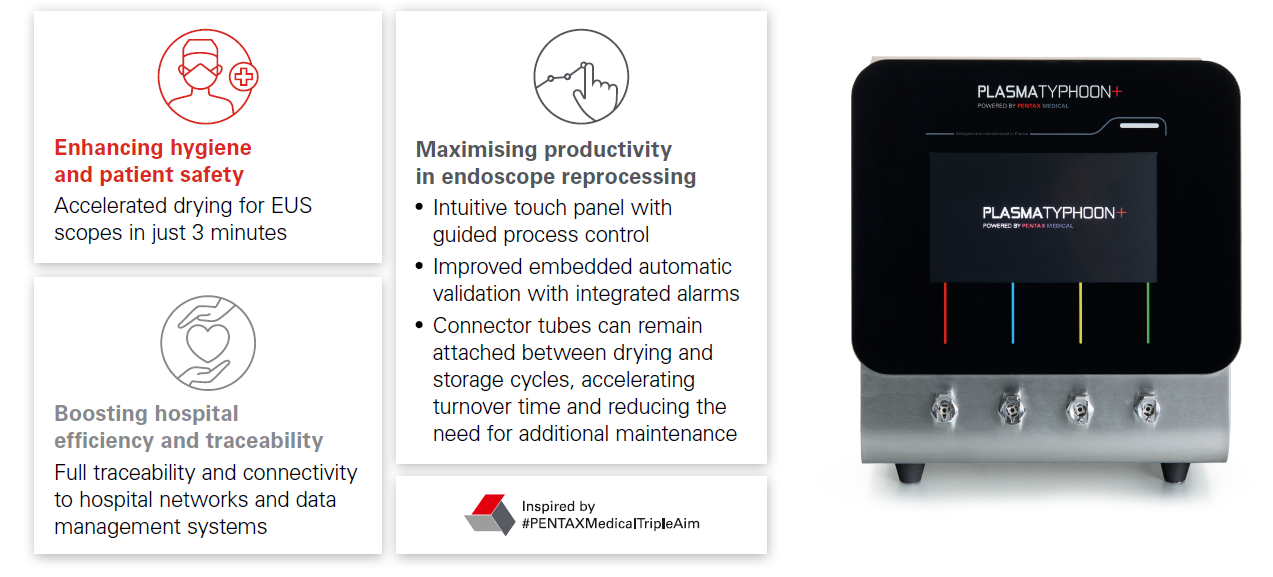
Making reprocessing procedures easier and safer
The new generation PlasmaTYPHOON™+ makes it possible:

Even more, PlasmaTYPHOON™+ provides additional support and safety in daily processes:
|  |
In combination with the PlasmaBAG™ storage solution, PlasmaTYPHOON™+ offers you safe and active endoscope storage with many benefits:

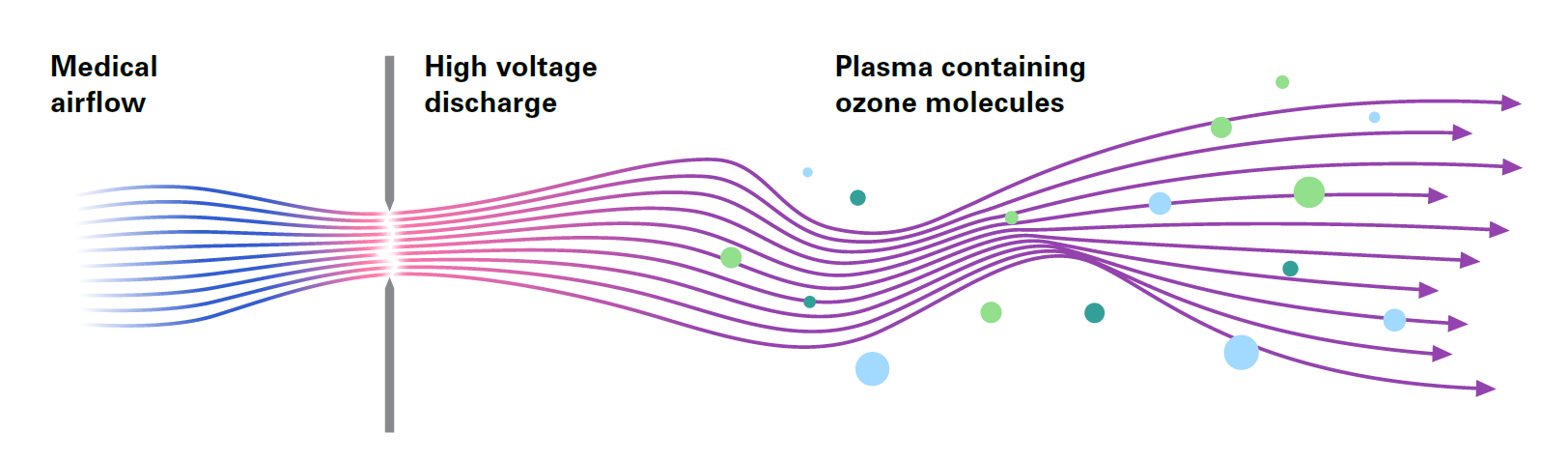
PlasmaBAG™: Sustaining the disinfected state with plasma
After the completely dried endoscope has been placed in the PlasmaBAG™, the bag is insufflated with plasma containing ozone molecules. In conjunction with complete drying, the active storage environment in the PlasmaBAG ensures the disinfected state of the endoscope is maintained for up to 31 days 2).
Caring for environment with PlasmaBAG ECO™
PENTAX Medical aims to support the UN Sustainability Development Goals. PlasmaBAG™ is one example of our continuous journey to sustainability:

PlasmaTYPHOON™+ and PlasmaBAG™ System:
What experts say about the system


PlasmaTYPHOON™+ and PlasmaBAG™ System:
Independently tested
PlasmaTYPHOON™+ and PlasmaBAG™ have been thoroughly tested by Eurofins Biotech Germande Laboratory having Cofrac accreditation.
The system is certified to comply with the EN 16442 standard for drying and storage of flexible endoscopes, contributing to improved hygiene and patient safety.

PlasmaTYPHOON™+ and PlasmaBAG™ System:
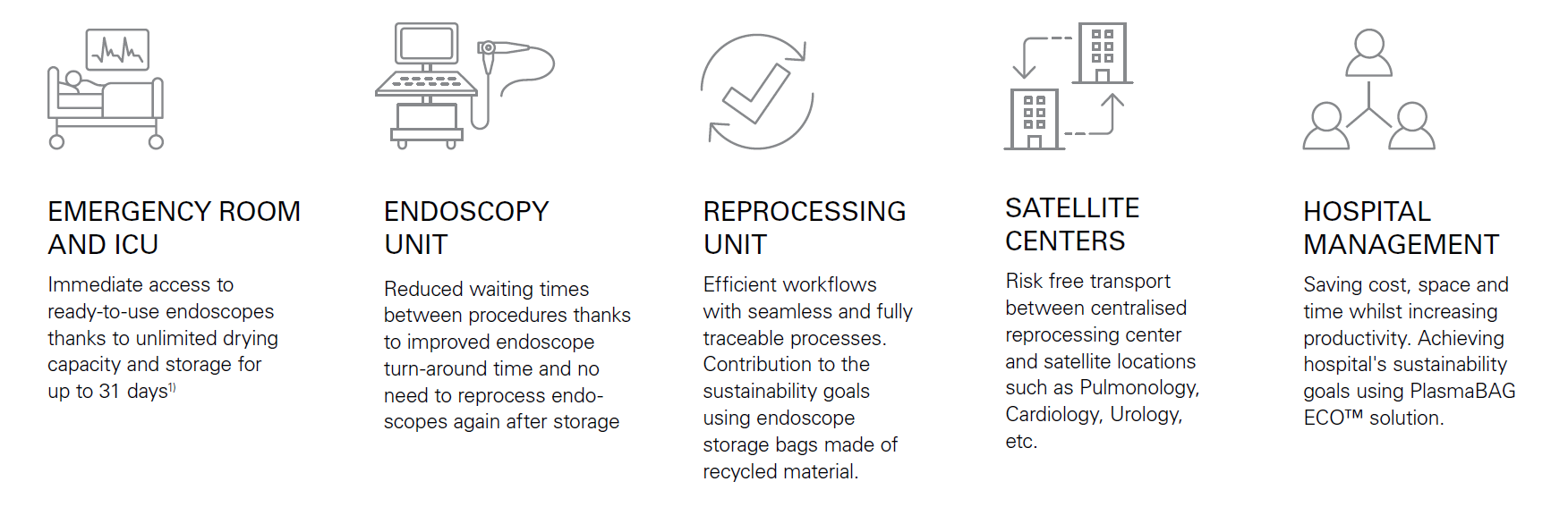
Supports every department in your hospital or clinic
The system fulfils your departments’ needs and can help you increase hospital efficiency and productivity. With the PlasmaBAG™ ECO, it offers physicians, nurses, reprocessing staff and hospital staff a solution that supports reaching your sustainability goals, as well:
To learn more about the benefits and functionality:
www.the-hygiene-solution-that-fits.com
Technical data
1) PlasmaTYPHOON™+ and PlasmaBAG™ ECO System:
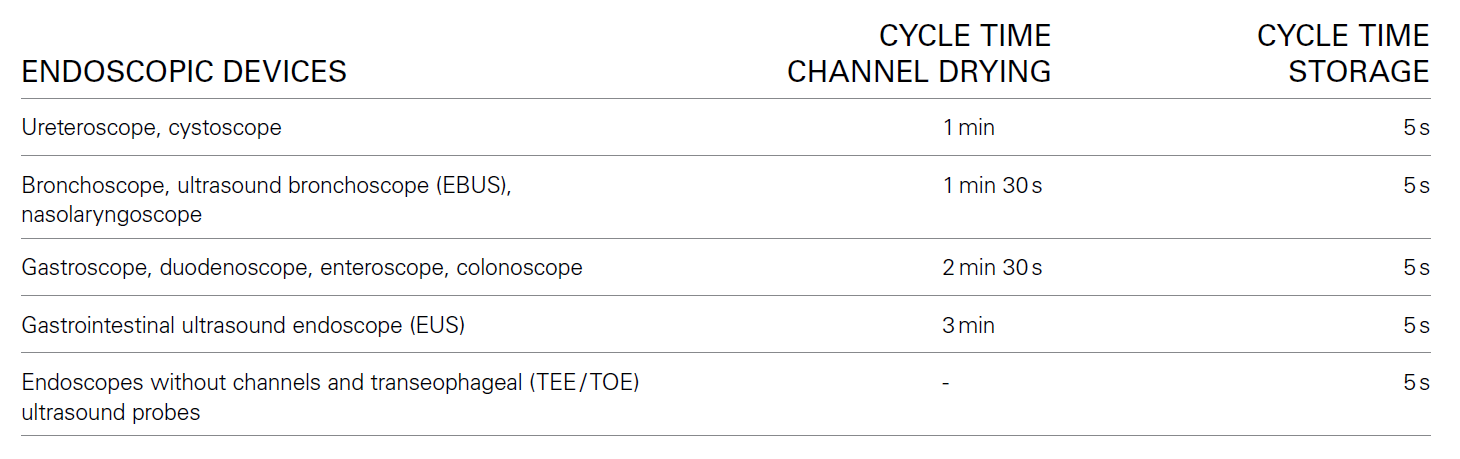
Ultra-fast drying and storage times
The system is fully compatible with all major endoscope brands.
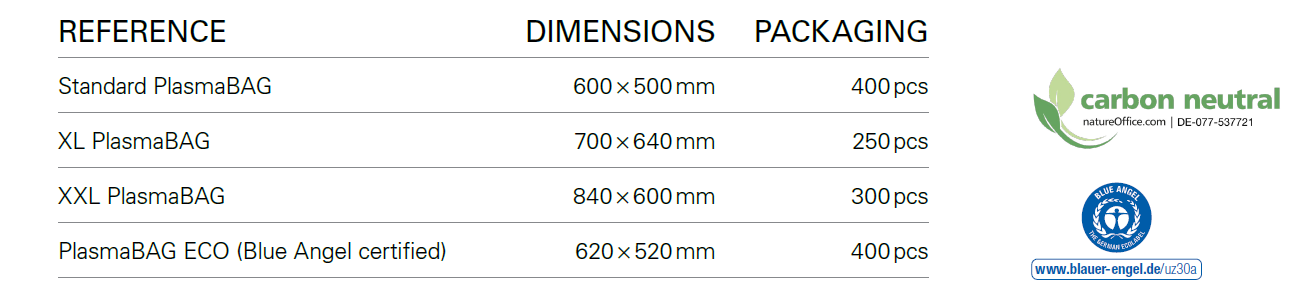
At a glance:
The PlasmaTYPHOONTM+ just got even better
What’s new with our premium solution PlasmaTYPHOON+?
References:
2)Validated for up to 744 storage hours (31 days) according to NF EN 16442 norm. The maximum storage time may be subject to local regulations on endoscope storage.
3) Until 2021, Project Togo has planted more than 1,500,000 new trees in total, binding approx. 400,000 tons of atmospheric carbon annually. www.natureoffice.com/en/carbon-offset-projects/project-togo
4) Equivalent trees and compensated volume of carbon according to calculation of L.E.S.S. FRANCE
PlasmaTYPHOON+
| Product |
|---|
| PlasmaTYPHOON+ |
| Power Requirements | Frequency | Dimensions | Net weight | Pressure of medical air at gas inlet | Minimal gas flow rate |
|---|---|---|---|---|---|
| (V) | (Hz) | (L x W x H) | (kg) | (Bar) | (l/min) |
| 110-240 | 50/60 | 280 x 260 x 300 | 10.7 | 3 | 60 |
Accessories
Please click on product model to view related accessories