Press Release

PENTAX Medical Europe launches PROfILE single-use cleaning brushes for endoscope reprocessing
Hamburg, 21 June 2017 – PENTAX Medical Europe announces the launch of PROfILE single-use cleaning brushes for endoscope reprocessing to the EMEA market. This range of disposable brushes has been designed to meet the high standards expected of PENTAX Medical, with a focus on patient safety and hygiene. In addition to its high quality endoscope offerings, PENTAX Medical is committed to providing a complete and hygienic solution for endoscopy that also includes hygiene consumables, accessories, and tools for advanced therapy.
The new PROfILE single-use brushes have been extensively tested in combination with PENTAX Medical endoscopes to meet the strict standards of the Association for the Advancement of Medical Instrumentation (AAMI) and FDA’s 2015 Final Guidance on Reprocessing Reusable Medical Devices. When used according to the Instructions for Use for the brush and the scope reprocessing, we are confident that the new single-use brushes help deliver reliable reprocessing results and enhance the performance of PENTAX Medical endoscopes.
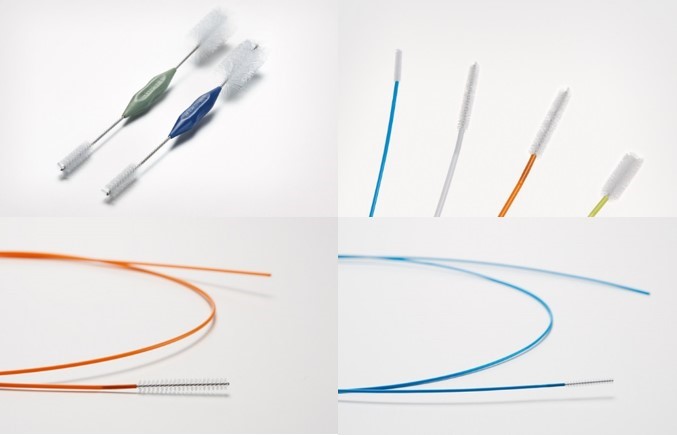
The new PENTAX Medical PROfILE single-use cleaning brushes.
Designed exclusively for PENTAX Medical endoscopes, the use of these dedicated brushes supports compliance with PENTAX Medical Instructions for Use and recommended reprocessing practices, according to guidelines issued by gastroenterology associations around the globe. ESGE and ESGENA guidelines recommend a switch from reusable to disposable brushes to allow for maximum effectiveness of cleaning and to avoid tissue carryover1.
The full portfolio of PROfILE single-use cleaning brushes from PENTAX Medical includes nine different competitively priced models, exclusively designed to cover most of PENTAX Medical’s endoscopes. The range includes six long channel brushes and three short channel brushes. A pull-through technique takes advantage of the bristles located at one end of the brushes to facilitate proper and complete cleaning.
In addition to the support it offers throughout the complete clinical pathway, PENTAX Medical has a strong focus on patient safety and hygiene, which is positioned at the forefront of its product design and development programs. Demonstrating this ongoing commitment to patient safety, the PROfILE single-use cleaning brushes have been designed to address reprocessing needs. These cleaning brushes are the first accessories in a line of hygiene-related products that will be released by the company over the next months. This new range of accessories is designed specifically for PENTAX Medical’s endoscopes, to support you in fulfilling the needs of hygiene departments, reducing the risk of infection and cross contamination, and increasing confidence in safety of the reprocessing outcome.
Rainer Burkard, President EMEA, PENTAX Medical commented, “Proper manual cleaning of flexible endoscopes is key to reliable high-level disinfection and infection prevention. At PENTAX Medical, we take patient safety and hygiene incredibly seriously. As the majority of the cleaning procedures are carried out by a combination of manual pre-cleaning and automated reprocessing we have focused on providing enhanced manual cleaning accessories. With our new range of PROfILE single-use cleaning brushes, we provide a tool that has been validated and verified for use with PENTAX Medical endoscopes to improve confidence in manual cleaning and support compliance with our instructions for use.”
About PENTAX Medical
PENTAX Medical is a division of HOYA Group. Its mission is to improve the standard of patient care and quality of healthcare delivery by providing the best endoscopic products and services with a focus on QUALITY, CLINICALLY RELEVANT INNOVATION, and SIMPLICITY. Through leading edge R&D and manufacturing, PENTAX Medical provides endoscopic imaging devices and solutions to the global medical community. Headquartered in Japan, PENTAX Medical has a worldwide focus and presence with R&D, regional sales, service, and in-country facilities around the globe.
For more information, please visit: www.pentaxmedical.com.
1. ESGE–ESGENA guideline: Cleaning and disinfection in gastrointestinal endoscopy Update 2008.DOI 10.1055/s-2008-1077722 Endoscopy 2008; 40: 939–957

